Chemistry Journal of Moldova
Physical chemistry and chemical physics
Author(s):
Field: Physical chemistry and chemical physics
Type: Research paper
Issue: 2021 Volume 16, no.2
Pages: 102-111
Thamer Adnan Abdullah, Tatjana Juzsakova, Rashed Taleb Rasheed, Ali Dawood Salman, Mohammademad Adelikhah, Le Phuoc Cuong, Igor Cretescu
Field: Physical chemistry and chemical physics
Type: Research paper
Issue: 2021 Volume 16, no.2
Pages: 102-111
Full Text (PDF): Download
Abstract (PDF)
Graphical Abstract: This paper deals with V2O5 nanoparticles adsorbents which were obtained by thermal pretreatment carried out by increasing the temperature between 90 and 750°C. In order to obtain more detailed information on the surface chemistry of the newly prepared nanoparticles, the characterisation was done by X-ray diffraction and scanning electron microscopy, Fourier Transform infrared spectroscopy and thermogravimetric investigation technique. The prepared nanoparticles were tested for methylene blue (MB) removal from modelled water solutions. The obtained results indicated that increased MB removal efficiency (93%) and adsorption capacity (27 mg/g) after 40 minutes of adsorption were obtained for V2O5 annealed at 500°C. The applicability and suitability of the two kinetic models were tested and the removal mechanism was proposed.
Graphical Abstract: This paper deals with V2O5 nanoparticles adsorbents which were obtained by thermal pretreatment carried out by increasing the temperature between 90 and 750°C. In order to obtain more detailed information on the surface chemistry of the newly prepared nanoparticles, the characterisation was done by X-ray diffraction and scanning electron microscopy, Fourier Transform infrared spectroscopy and thermogravimetric investigation technique. The prepared nanoparticles were tested for methylene blue (MB) removal from modelled water solutions. The obtained results indicated that increased MB removal efficiency (93%) and adsorption capacity (27 mg/g) after 40 minutes of adsorption were obtained for V2O5 annealed at 500°C. The applicability and suitability of the two kinetic models were tested and the removal mechanism was proposed.
Downloads: 138
Author(s):
Field: Physical chemistry and chemical physics
Type: Research paper
Issue: 2021 Volume 16, no.2
Pages: 91-101
Tatyana Rakitskaya, Tatyana Kiose, Lyudmila Raskola
Field: Physical chemistry and chemical physics
Type: Research paper
Issue: 2021 Volume 16, no.2
Pages: 91-101
Full Text (PDF): Download
Abstract (PDF)
Graphical Abstract: The effect of the nature and concentration of d-metal salts attached to synthetic zeolites NaA and KA on the kinetic and stoichiometric parameters of the chemisorption-catalytic oxidation of sulphur dioxide with air oxygen at ambient temperature was studied. It was found that the adsorption capacity of NaA zeolite relative to SO2 is 100 times higher than that of KA zeolite; the time of protective action of NaA and KA zeolites increases upon modification with transition metal salts and with an increase of their content in the compositions. It was shown that the formation of inner and outer sphere complexes and the relationship between them is determined by the nature and concentration of metal ions and by the nature of the carrier. It was proven that the chemisorption-catalytic process ends with the oxidation of SO2 to H2SO4.
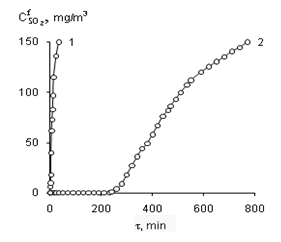
Graphical Abstract: The effect of the nature and concentration of d-metal salts attached to synthetic zeolites NaA and KA on the kinetic and stoichiometric parameters of the chemisorption-catalytic oxidation of sulphur dioxide with air oxygen at ambient temperature was studied. It was found that the adsorption capacity of NaA zeolite relative to SO2 is 100 times higher than that of KA zeolite; the time of protective action of NaA and KA zeolites increases upon modification with transition metal salts and with an increase of their content in the compositions. It was shown that the formation of inner and outer sphere complexes and the relationship between them is determined by the nature and concentration of metal ions and by the nature of the carrier. It was proven that the chemisorption-catalytic process ends with the oxidation of SO2 to H2SO4.
Downloads: 78
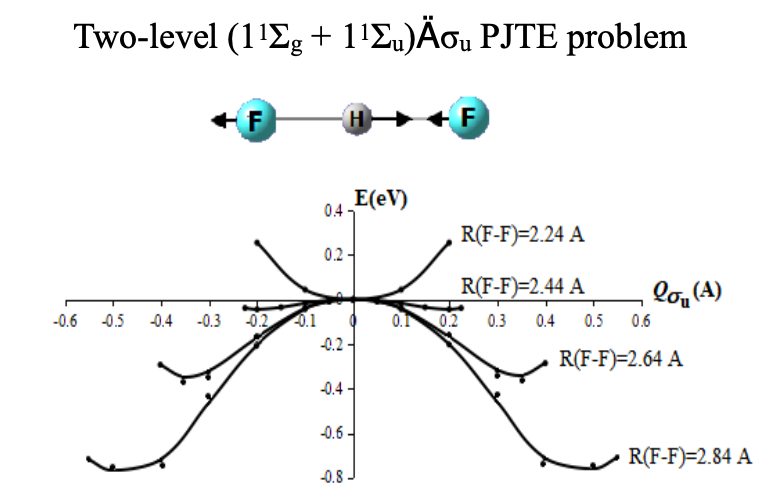
PSEUDO JAHN-TELLER ORIGIN OF THE PROTON-TRANSFER ENERGY BARRIER IN THE HYDROGEN-BONDED [FHF]- SYSTEM
Natalia Gorinchoy, Iolanta Balan, Victor Polinger, Isaak Bersuker
Field: Physical chemistry and chemical physics
Type: Research paper
Issue: 2021 Volume 16, no.1
Pages: 115-120
Full Text (PDF): Download
Graphical Abstract: The results of ab initio calculations of the adiabatic potential energy surfaces for the proton-bound [FHF]- system at different F-F distances have been rationalized in the framework of the vibronic theory. It is shown that the instability of the symmetric D∞h structure at increased F∙∙∙F distances and the proton displacement to one of the fluorine atoms is due to the pseudo Jahn–Teller mixing of the ground 1Σg electronic state with the lowest excited state of 1Σu symmetry through the asymmetric σu vibrational mode.

Downloads: 111
Author(s):
Field: Physical chemistry and chemical physics
Type: Invited paper
Issue: 2020 Volume 15, no.2
Pages: 7-28
Igor Povar
Field: Physical chemistry and chemical physics
Type: Invited paper
Issue: 2020 Volume 15, no.2
Pages: 7-28
Full Text (PDF): Download
Graphical Abstract: The main scientific achievements of great significance accomplished by Professor Ilie Fishtik at the University of Iowa and the Worcester Polytechnic Institute, in several fields of the physical chemistry as chemical thermodynamics, kinetics and heterogeneous catalysis were revealed and briefly analysed. Fundamental equations of chemical thermodynamics within the De Donder (stoichiometric) approach were reformulated in terms of a special class of chemical reactions, called as response reactions. Using this approach, the unusual behaviour of chemical equilibrium systems, to interpret the apparent contradictions to Le Chatelier principle and to discover hitherto unnoticed thermodynamic identities, was rationalised. The stabilities of chemical species were formulated in terms of a certain class of stoichiometrically unique chemical reactions and their thermochemical characteristics. A completely new approach for the generation and simplification of kinetic mechanisms for complex reaction systems was developed and applied. Based on a new type of reaction networks, referred to as reaction route graphs, a systematic method of analysis and reduction of a microkinetic mechanism was established and employed.
Downloads: 168
Author(s):
Field: Physical chemistry and chemical physics
Type: Research paper
Issue: 2020 Volume 15, no.1
Pages: 95-102
Olga Kazakova, Roman Ivannikov, Iryna Laguta, Oksana Stavinskaya, Viktor Anishchenko, Olga Severinovska, Ludmila Buyun
Field: Physical chemistry and chemical physics
Type: Research paper
Issue: 2020 Volume 15, no.1
Pages: 95-102
Full Text (PDF): Download
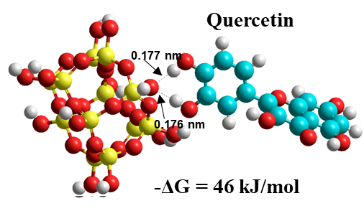
Graphical Abstract: The most common phenolic components of sixteen orchid extracts were identified using high performance liquid chromatography and laser desorption/ionization mass spectrometry. The interaction between these compounds and silica silanol groups was studied using quantum chemical calculations. Results show that the strength of the interaction of phenols with silica increased in the following order: ferullic, feruloylquinic and fertaric acids <kaempferol, apingenin <<сhlorogenic and caffeic acids, rhamnetin, quercetin, luteolin, epicatechin gallate. The common feature of compounds characterized by the strongest interaction with silanol groups is the presence of phenol ring with two neighbouring hydroxyl groups.

Downloads: 175
Author(s):
Field: Physical chemistry and chemical physics
Type: Research paper
Issue: 2019 Volume 14, no.1
Pages: 98-106
Nur Nadira Hazani, Yusairie Mohd, Sheikh Ahmad Izaddin Sheikh Mohd Ghazali, Nur Nadia Dzulkifli
Field: Physical chemistry and chemical physics
Type: Research paper
Issue: 2019 Volume 14, no.1
Pages: 98-106
Full Text (PDF): Download
Abstract (PDF)
Graphical Abstract: The inhibitive effects of 2-acetylpyridine 4-ethyl-3-thiosemicarbazone (HAcETSc) and dichlorophenyltin(IV) 2-acetylpyridine 4-ethyl-3-thiosemicarbazone (Sn(HAcETSc)PhenCl2) for mild steel in 1 M HCl solution at different concentrations were investigated using electrochemical measurements and scanning electron microscopy analysis. The result of electrochemical measurement found that the inhibition efficiency increased with inhibitors’ concentration. Moreover, it was shown that that Sn(HAcETSc)PhenCl2 had a better inhibitive effect than HAcETSc.

Downloads: 310
Author(s):
Field: Physical chemistry and chemical physics
Type: Research paper
Issue: 2019 Volume 14, no.1
Pages: 107-119
Yuri Mirgorod, Alexander Chekadanov, Tatiana Dolenko
Field: Physical chemistry and chemical physics
Type: Research paper
Issue: 2019 Volume 14, no.1
Pages: 107-119
Full Text (PDF): Download
Abstract (PDF)
Graphical Abstract: Aqueous micellar solutions of sodium dodecyl sulphate were investigated using X-ray scattering technique by a model-independent approach and dynamic light scattering in the concentration range 0.008 – 0.1 M. The obtained results are discussed in the framework of the concept of polyamorphous transition between ensembles of water clusters of low and high density levels. Polyamorphous transition accompanies the formation of dual structures of contact and separated by water micelles with different rates of diffusion.
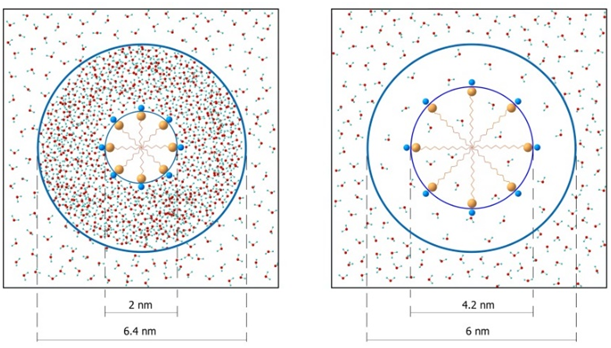
Graphical Abstract: Aqueous micellar solutions of sodium dodecyl sulphate were investigated using X-ray scattering technique by a model-independent approach and dynamic light scattering in the concentration range 0.008 – 0.1 M. The obtained results are discussed in the framework of the concept of polyamorphous transition between ensembles of water clusters of low and high density levels. Polyamorphous transition accompanies the formation of dual structures of contact and separated by water micelles with different rates of diffusion.

Downloads: 226
Author(s):
Field: Physical chemistry and chemical physics
Type: Research paper
Issue: 2019 Volume 14, no.1
Pages: 88-97
Ion Arsene, Natalia Gorinchioy
Field: Physical chemistry and chemical physics
Type: Research paper
Issue: 2019 Volume 14, no.1
Pages: 88-97
Full Text (PDF): Download
Abstract (PDF)
Graphical Abstract: The reaction cycle of H2O2 decomposition and O2 generation catalyzed by Fenton reagent was studied using density functional theory calculations. A four-stage mechanism for the oxygen production and the Fe2+ regeneration in the Fenton reaction is proposed based on the obtained results. It is shown that the O-O bond cleavage of coordinated H2O2at the first step of reaction does not lead to a free HO● radical. Instead, a highly reactive intermediate [FeIV(H2O)4(OH)2]2+ with two HO● radicals “trapped” in the complex is formed. The result of the next two reaction steps is the formation of the two HO2● radicals which can react on the triplet energy surface in order to produce O2 and a H2O2.
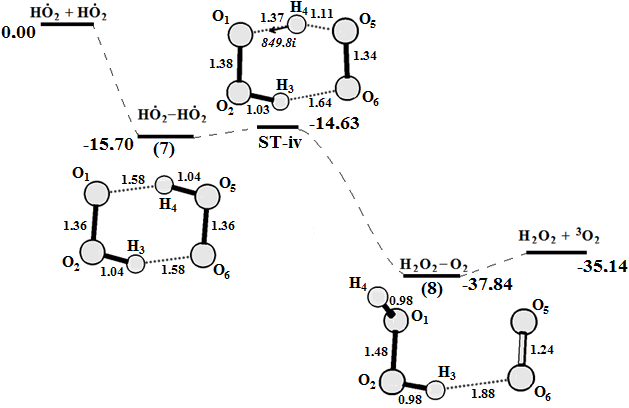
Downloads: 195
Author(s):
Field: Physical chemistry and chemical physics
Type: Research paper
Issue: 2019 Volume 14, no.1
Pages: 77-87
Viktoria Vorobyova, Margarita Skіba
Field: Physical chemistry and chemical physics
Type: Research paper
Issue: 2019 Volume 14, no.1
Pages: 77-87
Full Text (PDF): Download
Graphical Abstract: The protection performance of self-assembled layers (SALs) formed by apricot cake extract (ACE) on the surface of steel has been studied. It was revealed that the protection ability of the SALs is determined by the time of film formation on the steel surface. The maximal corrosion inhibition efficiency (about 93%) was obtained after 48 h process of film formation in the vapour phase of the apricot cake extract. The results of the electrochemical analysis revealed that the ACE modified steel showed better corrosion protection in conditions of periodic condensation of moisture.
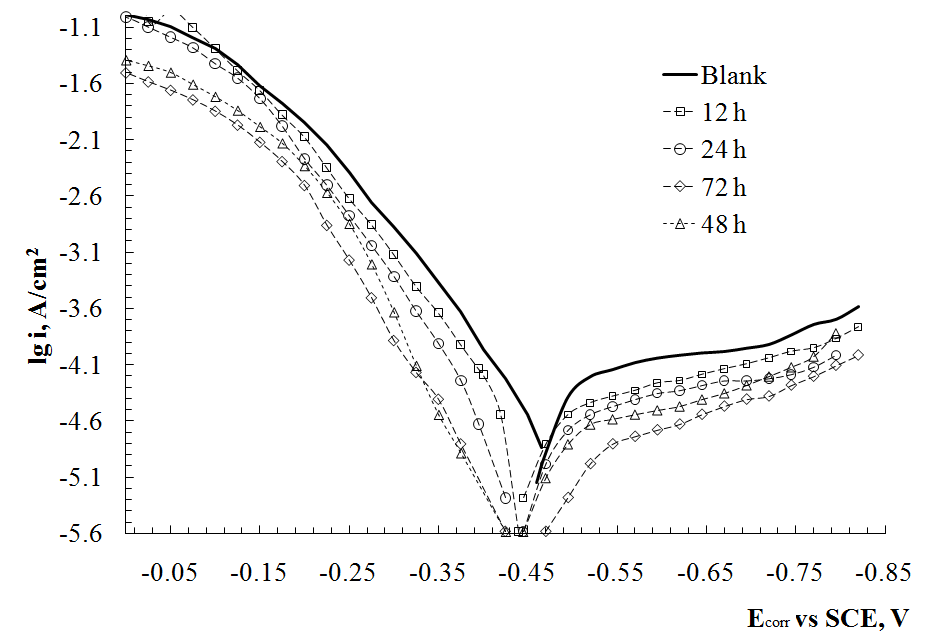
protection in conditions of periodic condensation of moisture.

protection in conditions of periodic condensation of moisture.
Downloads: 121
Author(s):
Field: Physical chemistry and chemical physics
Type: Research paper
Issue: 2018 Volume 13, no.2
Pages: 82-88
Kahina Hamza, Abdelkader Touati, Ahmed Ait-Yahia, Michel Baltas, Christiane André Barres, Saâd Moulay
Field: Physical chemistry and chemical physics
Type: Research paper
Issue: 2018 Volume 13, no.2
Pages: 82-88
Full Text (PDF): Download
Graphical Abstract: The present work provides an insight into the effect of the nature of surfactant (cationic, anionic), a component of water- and oil-borne microemulsions, on the reaction rate of 1,3-dipolar cycloaddition of C,N-diphenylnitrone with acrylonitrile. The electrostatically attractive character of the cationic surfactant, would bring the reactants closer to each other; hence, a rate enhancement would ensue, particularly within the water-rich zone. Besides the fact that acrylonitrile played a dual role, as a component of the microemulsion and a dipolarphile in the cycloaddition reaction, made the work-up advantageously sound. Additionally, the increase in reagents molar ratio was found to promote higher reactivity.
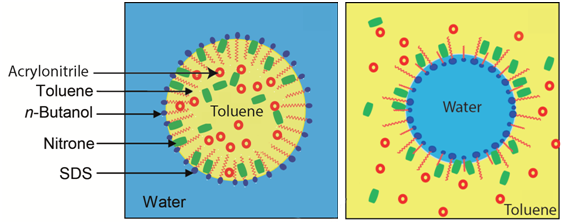

Downloads: 135






